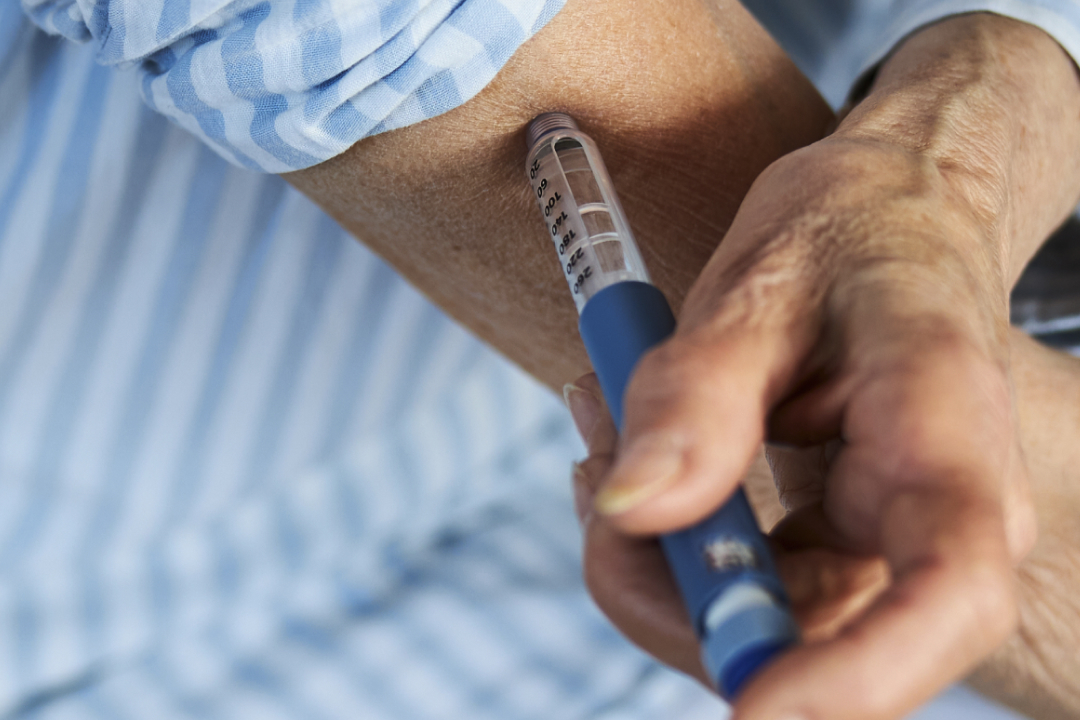
Access to GLP-1 receptor agonists (GLP-1 RAs), like semaglutide (Ozempic) and liraglutide (Saxenda), has transformed healthcare for many patients, improving the management of type 2 diabetes and offering new solutions for weight loss. These advancements are enabling better health outcomes for millions, showcasing the potential of innovative treatments to address chronic conditions effectively.
That said, there has been an unprecedented surge in demand for these devices that have resulted in significant challenges regarding supply. Beyond its immediate implications on patient care, this surge is disrupting the medical device market, particularly the supply chain for drug delivery systems like pre-filled pens and auto-injectors. Let’s take a look at these risks in detail and assess the wider implications across the industry.
Rising demand for GLP-1 injectable devices fuels new challenges
The popularity of GLP-1 RAs for managing type 2 diabetes and, increasingly, for weight loss has fuelled demand for injectable drug delivery systems. Pre-filled pens and auto-injectors, already integral to diabetes care, are now required in greater quantities than ever. However, scaling with this unexpected surge in demand has proven to be difficult due to a number of key challenges.
- Supply chain complexity: The pharmaceutical supply chain already has a very complex structure with a mixture of active pharmaceutical ingredients (APIs) and biocompatible materials like medical-grade stainless steel and advanced polymers. This unexpected surge in demand has put a strain on this supply chain, forcing engineers to navigate intricate logistics and sourcing challenges while balancing the precision and quality that are critical for components like springs and wire forms used in auto-injectors.
- Manufacturing bottlenecks: Automated assembly lines must handle complex components with tight tolerances to meet the high-quality standards of the medical sector. The intricate geometries of wire forms and pressed components can challenge production repeatability and increase scrap rates, making scalability a complex task.
- Regulatory pressures: As regulatory bodies push for zero tolerance on particulates and bioburden, manufacturers need to maintain both high output and uncompromised quality. Trying to dramatically scale production whilst remaining compliant with regulations like the UK Medical Device Regulations (UK MDR), EU Medical Device Regulation (EU MDR) and global standards (ISO and ICH) can be a very difficult task.
The risks tied to the surge in GLP-1 demand
The high demand for GLP-1 receptor agonists is creating ripple effects across the medical industry, exposing weaknesses in supply chains and presenting significant challenges for healthcare providers and manufacturers alike. The implications span patient care, the medical device market, and broader resource management, each of which faces distinct pressures.
Implications for patient care
The surge in demand for GLP-1 RAs has already had an impact on patients relying on these devices for their treatment. Patients with type 2 diabetes are struggling to get access to these drugs, with guidance being published by the NHS to prescribe alternative glucose management medications. Additionally, as resources are allocated toward manufacturing GLP-1 delivery devices, there is a risk of reduced availability of other essential medical products, potentially compromising care in other therapeutic areas.
Disruption to the medical device market
The focus on producing GLP-1 delivery devices is disrupting the broader medical device market. Manufacturers are reallocating resources to meet the unprecedented demand for pre-filled pens and auto-injectors, delaying the development and production of other critical devices such as reusable inhalers and diagnostic tools. There has also been a rise in counterfeit GLP-1 receptor agonists that pose a risk to patients, requiring greater attention from regulatory bodies that could be spent elsewhere.
Strained resources and rising costs
The rapid increase in production for GLP-1 delivery devices is placing a heavy strain on manufacturing resources, including materials, labour and production capacity. High demand for biocompatible polymers, medical-grade metals, and precision components is exacerbating supply chain pressures, while rising material and tooling costs are inflating overall production expenses. These costs are often passed down to healthcare providers and patients, potentially impacting the affordability and accessibility of care.
Manage unprecedented demand with intelligent manufacturing processes
The unprecedented demand for GLP-1 receptor agonists has placed immense strain on the medical device market, but solutions are within reach. By embracing strategic scaling practices, bolstering supply chain resilience and incorporating design for manufacturability principles, manufacturers can rise to the occasion and ensure both quality and efficiency.
Delve deeper into these solutions, exploring how manufacturers can leverage cutting-edge techniques and industry expertise to overcome scalability challenges, reduce rejection rates and maintain compliance with stringent regulatory standards.