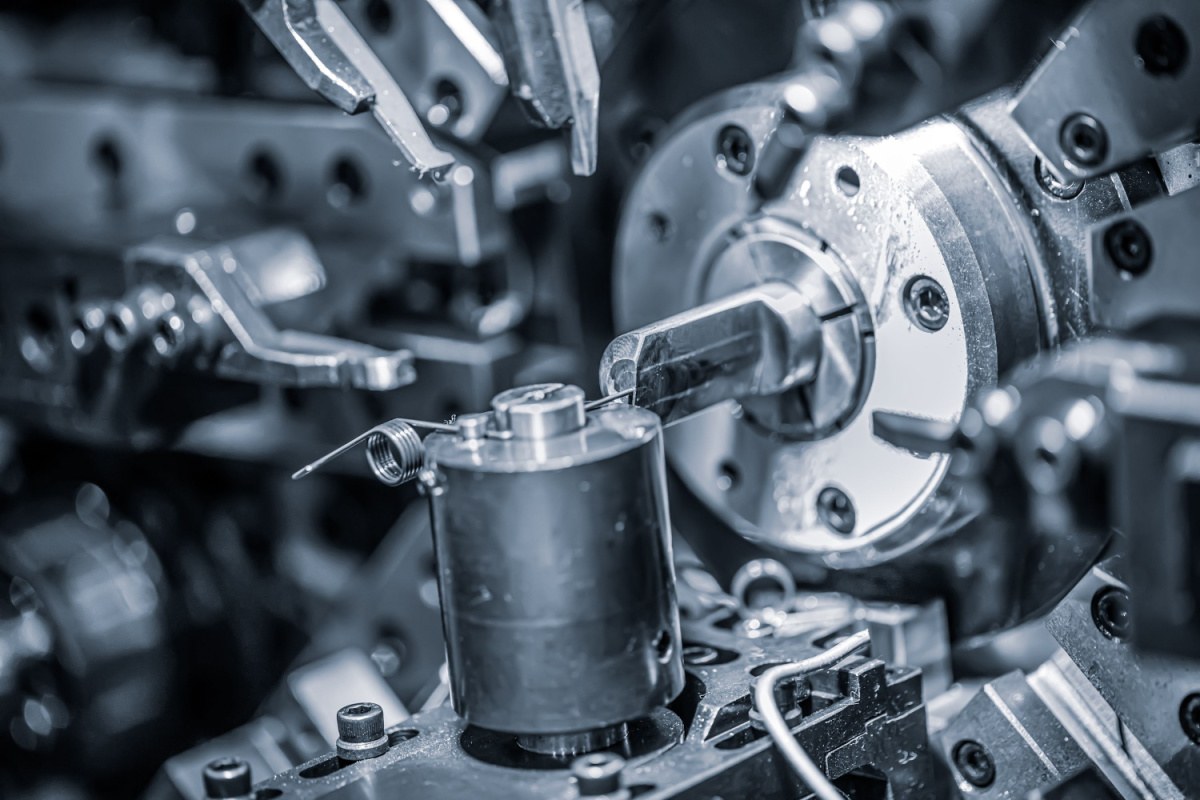
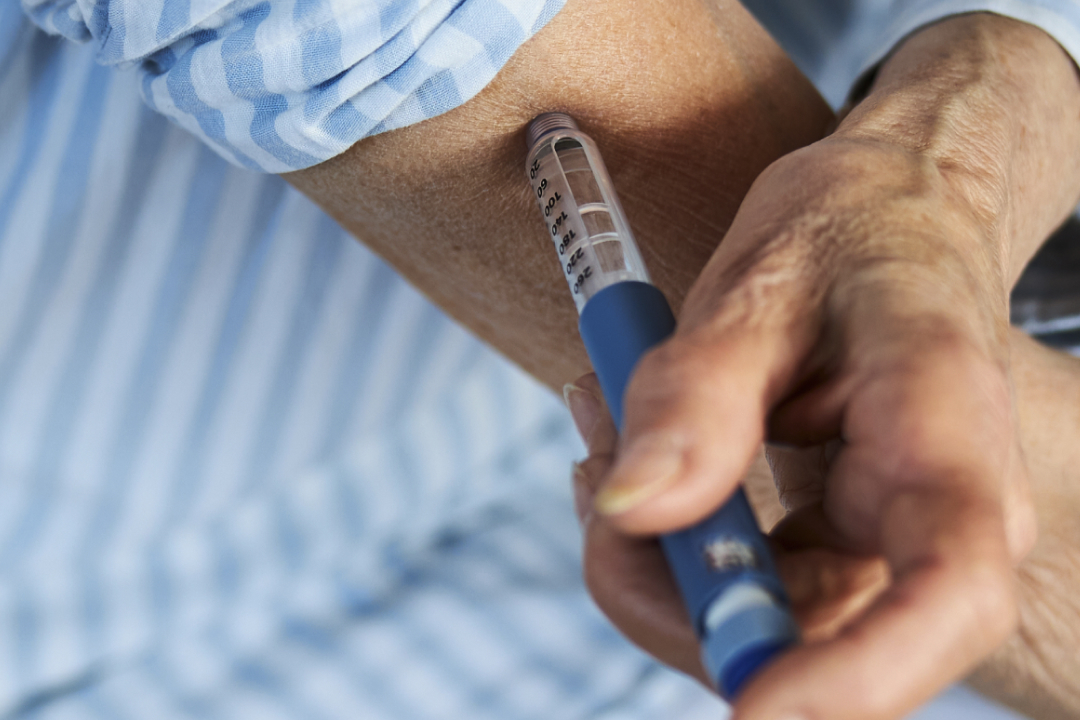
The production of GLP-1 drug delivery devices requires extreme precision to meet the stringent quality standards whilst maintaining a steady production output. But with the demand for these devices surging over the past year, manufacturers now need to look towards scalability as well as high production yields.
One step in achieving this scalability is through minimising scrap rates, but achieving consistency across high-volume production can be challenging as even minor variances can result in defective components or non-compliance.
To help scale, manufacturers need a clear understanding of how to minimise scrap rates in GLP-1 device production, focusing on precision engineering, lean manufacturing and quality control to ensure efficiency and reduce waste.
Precision engineering - the foundation of consistency
Precision engineering plays a crucial role in maintaining consistency during the assembly of GLP-1 drug delivery devices. Components such as springs, wire forms and needle-based systems require micrometre-level tolerances to ensure proper device functionality, including accurate dose delivery and reliable mechanical performance (ISO 11608 Series).
One critical example is the use of advanced micropressing techniques to manufacture ultra-thin, high-strength components, as emphasised in ISO 11608-1:2022. By leveraging state-of-the-art tools and materials like nitronic stainless steel, manufacturers can produce components that maintain dimensional stability even under high-stress conditions.
For instance, needle-based injection systems governed by ISO 11608 standards demand rigorous adherence to design specifications to ensure safety and performance. Variances in needle alignment or spring tension can lead to inaccurate dosing or device failure, emphasising the need for precision at every stage of production.
Investing in robust tooling and modular die sets further enhances precision by enabling consistent part replication. These tools reduce the likelihood of deformation and misalignment during assembly, ultimately lowering rejection rates and improving yield.
Continuous improvement - integrating lean manufacturing practises
Lean manufacturing principles and strategies can directly minimise waste whilst enhancing efficiency at the same time. One of these principles is process automation and plays a key role in streamlining the assembly of pre-filled pens and auto-injectors by reducing handling errors and ensuring consistent device performance. These automated systems improve production yield whilst maintaining compliance with regulatory standards.
Another principle is Design for Manufacture (DfM), where devices are designed with efficiency in mind. This helps reduce complexity and material waste through steps like optimising component geometries, minimising the number of assembly steps and selecting durable, compliant materials. Addressing manufacturability at the design stage also ensures that production remains scalable and cost-effective without compromising quality, which is particularly important in light of the GLP-1 situation.Quality control - ensuring uniformity at scale
Advanced quality control (QA) methods are essential for maintaining uniformity in high-volume production. Manual visual inspection methods will likely struggle to keep pace with the rapid output of GLP-1 device manufacturing, making automated systems increasingly vital.
Vision inspection systems, for example, use high-resolution imaging to detect surface irregularities, misaligned components or improper assembly. Paired with an automated system, manufacturers can identify defects at scale and achieve real-time corrections that reduce variability in components. By implementing proactive QA methods with automated systems, manufacturers can directly reduce scrap rates and improve overall process reliability.Optimise each step of your manufacturing to lower scrap rates
Minimising scrap rates in the production of GLP-1 drug delivery devices is critical for ensuring operational efficiency, reducing costs and maintaining compliance with regulatory standards. By prioritising precision engineering, implementing advanced quality control methods, and committing to lean processes, manufacturers can address the challenges of high-volume production whilst reducing waste.
As demand for GLP-1 devices continues to grow, investing in these strategies will benefit manufacturers and contribute to the broader goals of sustainability and patient safety. By refining processes and embracing innovation, the industry can meet the increasing demand without compromising on quality or efficiency. To find out more about how you can optimise your manufacturing processes to lower scrap rates, download our guide below.